
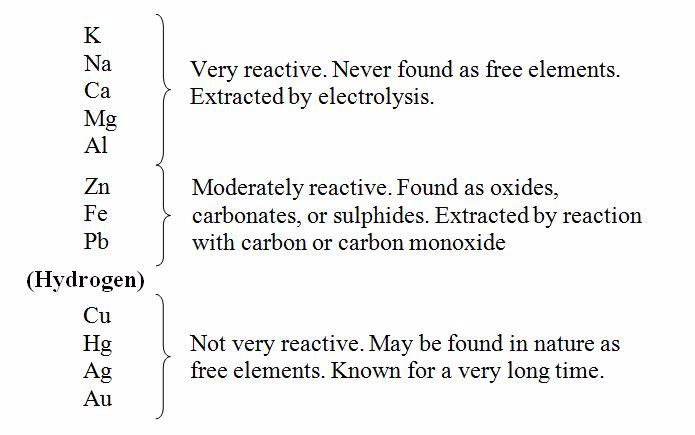
Post memes/jokes in /r/chemistrymemes and /r/chemistryjokes. Explanation: Notice the metals locations in the periodic table.
#Metal reactivity table series
Any such posts will be deleted.Īsk education and jobs questions in the current weekly topic. The reactivity series is a series of metals, in order of reactivity from highest to lowest. So, by reactivity series, you can tell which metal will displace another metal.
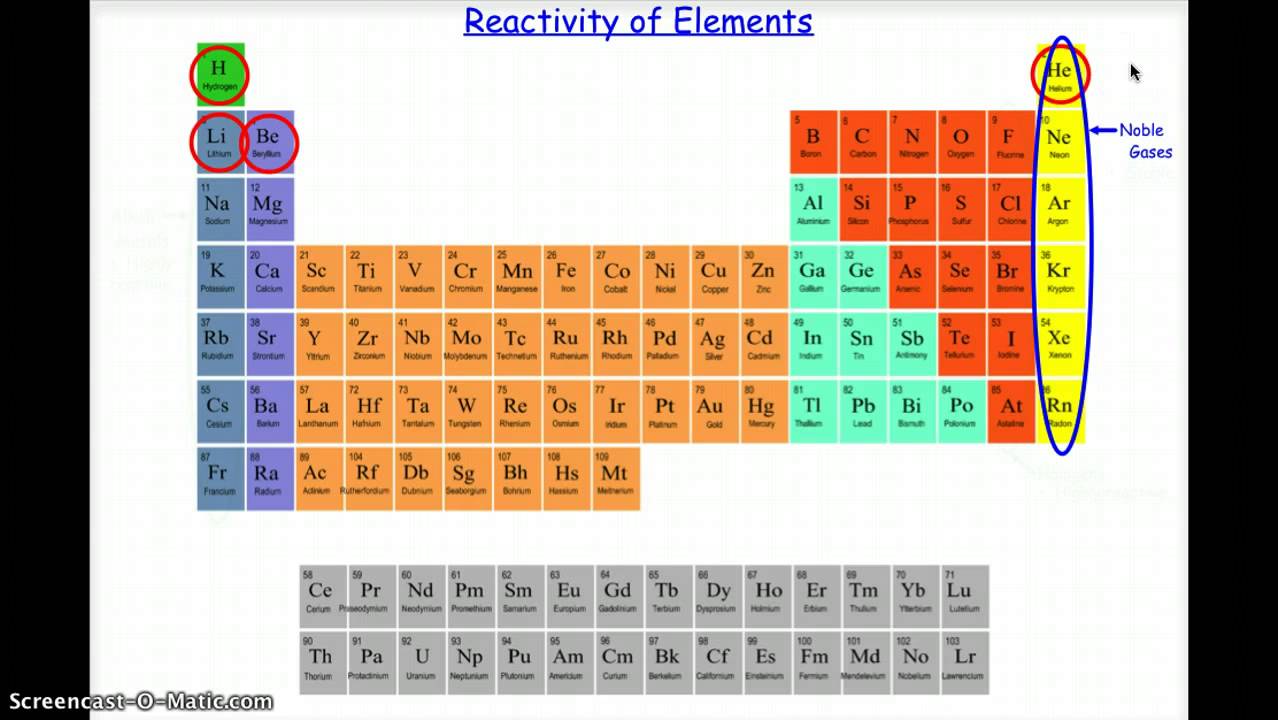
If you're looking for a more concentrated, advanced discussion of chemistry topics among professionals and grad students, check out /r/Chempros.īefore asking "What chemical is this?" see this chart. In displacement reaction Displacement reactions are those reactions in which more reactive metal displaces less reactive metal from its salt. Reactions of metals with water When a metal reacts with water, a metal. While metals from magnesium to lead can react with acids. Hydrogen and carbon are shown for comparison. Metals from potassium to calcium are highly reactive and even react with water. Click here for the OSHA chemical data site and here for a multicompany MSDS aggregate search. The table summarises some reactions of metals in the reactivity series. If you spill/injure yourself contact medical professionals and read the MSDS, do not post to this reddit. Metals in the second class are slightly less active. These metals are found exclusively in Groups IA and IIA of the periodic table. Given a table of metal activity you can identify which metal will be displaced. The most active metals are so reactive that they readily combine with the O 2 and H 2 O vapor in the atmosphere and are therefore stored under an inert liquid, such as mineral oil. Yes links to blogs, images, videos, comics, and infographics are okay especially if they are on your personal website. Reactivity of Metals, Metal Displacement and the Activity Series, redox. No physorg, sciencedaily, or other press release aggregator spam!

#Metal reactivity table license
If a caption or explanation is included this helps, but please use your discretion.īefore asking about chemical drawing/illustration programs, look at your school's IT/software website and see if they provide an institutional license of ChemDraw (hint: if they have a chemistry department, they will) Likewise, simple pictures of uninteresting and garden variety chemistry-related things are not appreciated.

No memes, rage comics, image macros, reaction gifs, or other "zero-content" material. I predict that calcium will be the most reactive because of its position in the periodic table and copper will also be the least reactive. However, academic discussions on pharmaceutical chemistry and the science of explosives are permitted. Solid nonmetals are also very brittle.Rules: Violating a rule will result in a ban.Īsk homework, exam, lab, and other undergraduate-level questions at ChemicalForums otherwise it will be deleted.ĭiscussions on illicit drug synthesis, bomb making, and other illegal activities are not allowed and will lead to a ban. Metals, therefore, become more reactive as they are located further to the left on the periodic table. A nonmetal is typically dull and a poor conductor of electricity and heat. Metals are also malleable (they can be beaten into thin sheets) and ductile (they can be drawn into thin wires). The electrochemical series, also known as the activity series, is a list of metals listed in order of decreasing reactivity or in order of decreasing ease of oxidation. A metal is a substance that is shiny, typically (but not always) silvery in color, and an excellent conductor of electricity and heat.


 0 kommentar(er)
0 kommentar(er)
